As the Product Manager for Pharsight’s Phoenix platform, I would like to announce a couple of key product updates: 1. In September, Pharsight will be releasing a new product: Phoenix QT+ to support cardiac safety analysis. Last year Pharsight hired Dr. Christine Garnett, the FDA’s cardiac safety expert and she has led a project to build an automated solution to perform standardized QT analysis and replicated the FDA’s approach. This the first commercial solution like this in the industry and we believe it will greatly streamline the analysis and make it now possible for clinical pharmacology to perform this analysis in compliant environment. As with other Phoenix product, Phoenix QT+ will also connect to the PKS database to create a full audit trail with version control of all QT analysis. To allow our clients to benefit immediately, it can be installed as an add-on to the current PHX 1.3 suite. A new category for Phoenix QT+ will be added to this forum so please make sure to sign up if you would like participate in discussions and learn more about this product. Stay tuned for upcoming product launch information. 2. Pharsight has been working very hard on the Phoenix 1.4 release, which will include updates to Phoenix WinNonlin (mostly Bioequivalence tool), Connect (Watson LIMS connector), NLME (dynamic memory allocation) and AutoPilot (increase performance). This version will be released in the first half of 2014. Please stay tuned and I will provide more information as we get closer to the launch date for Phoenix 1.4. Ana Henry Product Manager, Phoenix Pharsight
Hi Ana, Any news on the release of version 1.4 Christian
The development team is actually on working on the final stages of the 1.4 release and we anticipate the 1.4 release will be available to users in August. I believe Ana intends to have some Webex presentations around this time however if you have some specific questions about fixes/new features please don’t hesitate to ask. Simon
The release of Phoenix 1.4 is targeted for August and we will do a webinar on the new features in September. Key features in the upcoming release of Phoenix 1.4 include: 1. Support for Windows 8.1 Operating System and Windows Server 2012 2. Redesigned and extended documentation 3. Additional options for importing datasets into Phoenix 4. New inverse-t and inverse chi-square distribution functions within the Data Wizard 5. Added function to apply significant digits within the Data Wizard 6. Improvements in the Bioequivalence Wizard (Phoenix WinNonlin) to meet regulatory guidelines 7. Many Phoenix NLME improvements with memory usage and usability 8. A new Watson LIMS Plug-in in Phoenix Connect that allows the user to connect to a Watson LIMS database, extract study data, and to create a worksheet that is ready for analysis 9. Improvements to CDISC capabilities within Phoenix Connect by better handling of datasets formatted in SDTM and SEND during import and export 10. A new CDISC Data Preparer object in Phoenix Connect that creates an analysis-ready dataset from CDISC-formatted data 11. Phoenix QT+ is now installed as part of Phoenix 14 and has a new object that allows users to compare CQTc models Ana
I want to expand a little on what Ana posted to help people producing test and migraton plans. Phoenix 1.4 now includes support for Windows 8.1 (32- and 64-bit) operating system using a keyboard and mouse, both Professional and Enterprise editions. A new server operating system (Windows Server 2012 R2) is also supported. However, it will not support Windows 8.1 RT (Mobile) nor on a tablet. Phoenix 1.4 no longer supports and cannot be installed on; Windows XP, Windows Vista, or Windows Server 2003. Pharsight support for Phoenix 1.3 will officially end 18 months after 1.4’s release so if you haven’t done so already you should be planning to move from this version by the end of 2015. Simon
Hopefully the forum members have already received the mailshot about the forthcoming webinars to introduce Phoenix WinNonlin,Simon.
Topic: What’s New with Phoenix 1.4
Date/Time: Monday, November 10, 2014| 1:00 PM EST/10:00 AM PST (30 Minutes)
Speaker: Ana Henry, Director of Product Management
Description: Phoenix— the premier software platform for the management, analysis, and reporting of PK/PD data— has been enhanced with features that improve performance and increase usability. With this update, you can more efficiently share precilinical and clinical knowlwedge throughout your organization and track a drug through the development lifecycle via secure and consistent workflows.
and on the 13th Nov.
Phoenix WinNonlin Users: Take Advantage of All Phoenix Capabilities
will show you how to create Create Phoenix workflows that can execute scripts for commonly-used pharmacometrics tools such as NONMEM ®, R, and SAS ®.
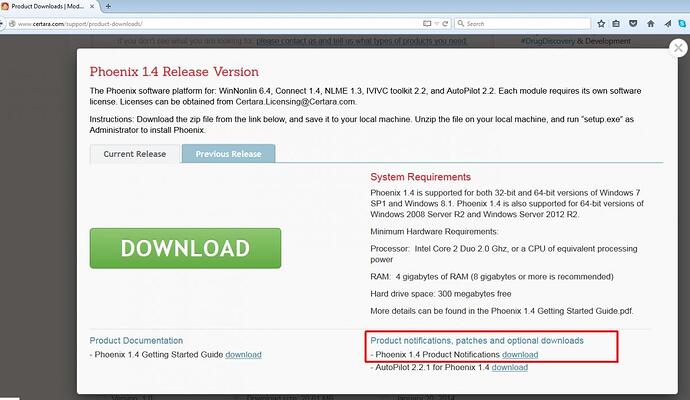
The Phoenix 1.4 release is now officially available on the Support page;
http://www.certara.com/support/product-downloads/
Please be sure to review the Release notes to get the most out of this new version.
Simon.
Dear team.
I am not quite sure that I am posting at the right place. Therefore, do not hesitate to move my post, if necessary.
I naturally hope my question is simple to answer, it is simple to ask.
We over here naturally rely on our template (as adapted to the project) during our evaluations, which was tested and checked and validated and is quality controlled …
Simply due to a lack of time we only now plan and prepare for the switch to WNL 6.4 and it is a relevant question for us (and me who will be doing most of the work in particular), whether it is possible to give a broad statement that “if your workflow based on standard objects (Data Manipulation Tools, NCA, BE) worked in 6.3 it will also work in 6.4.”
“With the exception that the results for the sequence effect will be correct if calculation is conducted in line with the requirements of the EMA ;)”
Is there any chance that such a broad statement (by myself or Certara, as a man can dream) is possible and will be acceptable for inspectors from your point of view?
Best regards,
Steven.
P.S.: is there a preview button here. I am sure there always was a preview button :huh:
Hi Steven,
It’s as good a place to ask as any, I’m not sure I’ll be able to give exactly the answer you are seeking however I think something along the lines of;
“if your workflow based on standard objects (Data Manipulation Tools, NCA, BE) worked in 6.3 it will also work in 6.4, excepting any changes/corrections that are noted in the 6.4 release notes”.
you already gave an example of one such exception;
"With the exception that the results for the sequence effect will be correct if calculation is conducted in line with the requirements of the EMA "
So the short answer would be that even with such a statement there would be a requirement upon your group to check the release notes, in particular the Issues Corrected section. With regard to Data tools one I would draw your attention to is;
‘Search’ returns the correct position based on a 1-based index (QC 11712): For example, search(“This is a test”,”s i”,0) will return position 4
since I have had some group that were using this Column Transformation and had to correct the count by one, since previously it was starting from 0 which was not consistent with the other functions.
Another way to check for these differences would be to re-run a small part of your previous validation test cases that you feel is a representative example. e.g. re-run one or two datasets that you used in v6.3 and confirm with a QC that you are are getting the same results. This more limited ‘risk-based’ assessment of your templates should enable you to migrate faster than a complete re-validation.
Oh and of course do check you’ve applied the latest patches by selecting from
http://www.certara.com/support/product-downloads/
View details next to the release you are interested in
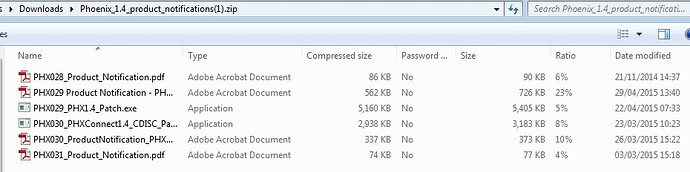
and then find the link to a Zip of Product Notifications etc. that will get updated with every patch and therefore grow in size - currently it is about 8.9Mb and looks like this;
Does that help?
Simon.
PS and yes there is a Preview post when you choose ‘More Reply Options’, there is also an Edit button if you realise you need to change something after submitting.
Hi Simon.
Thanks a lot for taking the time to answer.
I think that is exactly in line with what I expected and thought by myself.
Objects “stay the same” unless they underwent changes as documented in the release notes.
I am quite confident that we can work with that, also to reduce our “re-validation” to some representative examples or to a focus on the affected parts.
Well. more to write in the risk assessment verbiage but less and more helpful work during the actual change.
Aside: we agreed that we can make use of the “search” for a very company specific problem during transfer from Data Management to Biometrics instead of a larger change in the data management processes just yesterday. So, that is a nice example.
Best regards,
Steven.
P.S.: ah, “Attachments etc” is the way to go. Found the preview funtion.